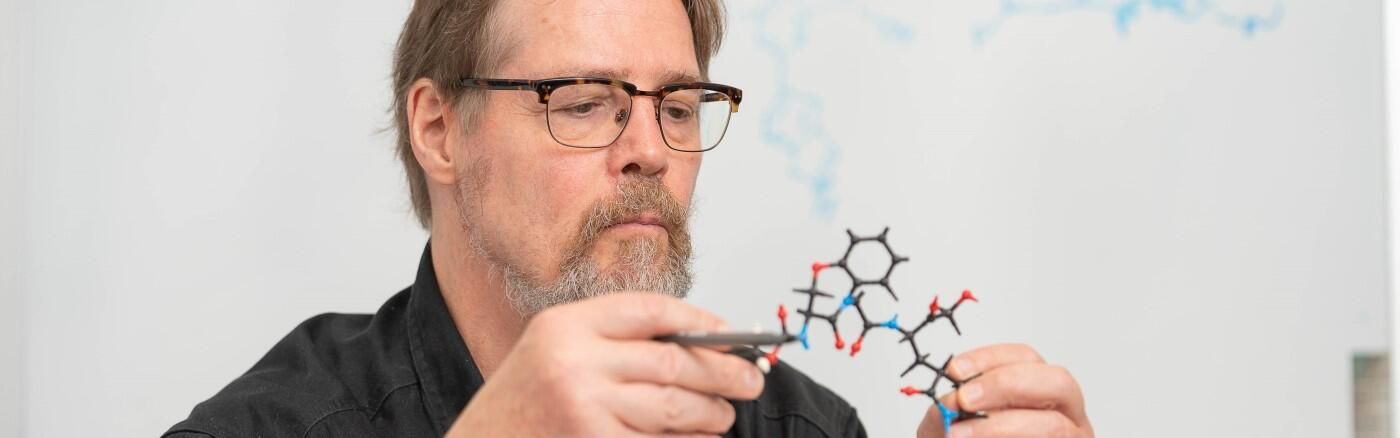
Scientists at The Wistar Institute Discover Novel Series of SARS-CoV-2 Mpro Inhibitors for Potential New COVID-19 Treatments

-
CONTACT:
-
Darien Sutton
PHILADELPHIA — (Oct. 8, 2024) — New research from The Wistar Institute’s Salvino lab — led by professor Joseph Salvino, Ph.D. — has identified a novel series of SARS-CoV-2 Mpro inhibitors that may lead to potential new COVID-19 treatments that, according to preclinical testing, effectively inhibits COVID-19 and synergizes with existing anti-COVID therapies. Their new discovery is detailed in the paper, “Design of novel and highly selective SARS-CoV-2 main protease inhibitors,” published in the journal Antimicrobial Agents and Chemotherapy.
Despite effective vaccines approved for use worldwide, COVID-19 continues to contribute to mortality and morbidity — an issue compounded by the problems of vaccine & therapy access. However, the existing drug designs in use for COVID-19 therapy lend themselves to drug interactions and the risk of incomplete viral inhibition.
To address this problem, Salvino — a medicinal chemist at Wistar — led a drug discovery team with the goal of improving upon the existing Mpro inhibitor design, an approach to viral therapy that seeks to prevent both viral replication and mutation-based drug resistance by targeting a component of the virus that regulates its ability to spread. And because Mpro is not an easy-to-mutate biological feature like a spike protein, inhibiting Mpro can help retain antiviral effectiveness even between different variations.
The team used a drug discovery technique that applied an “acyloxymethyl ketone electrophilic warhead” — in essence, a molecule designed to identify the important binding regions that a drug candidate compound would interact with. Using their drug discovery process, Salvino and the team identified a novel series of Mpro inhibitors with greater selectivity — that is, more reliable at producing an inhibitory effect — than the existing Mpro inhibitor for COVID-19 on the market.
The group’s novel compounds successfully inhibited viral replication in vitro against three different COVID variants, including within lung tissue. The compound also synergized (i.e., achieved greater-than-the-sum-of-its-parts strength) with other existing antivirals in fighting the virus. In the preclinical testing, no apparent toxicities were observed — a positive indication of the compound’s safety.
“We’re very excited to have identified such a promising new pathway for developing future therapies,” said Salvino. “As we continue to refine the chemistry through further testing and optimization, we look forward to achieving improved potency in anti-coronaviral therapies.”
Co-authors: Adi N. R. Poli, Ian Tietjen, Nitesh K. Nandwana, Joel Cassel, Troy E. Messick, Emery T. Register, Frederick Keeney, Luis J. Montaner, and Joseph M. Salvino of The Wistar Institute; and Rajesh Rajaiah, Atul K. Verma, Kabita Pandey, Arpan Acharya, and Siddappa N. Byrareddy of The University of Nebraska Medical Center.
Work supported by: NIH grants S10OD030245 and P30CA010815; Canadian Institutes of Health Research grant CIHR PJT-153057; and Commonwealth of Pennsylvania Special Initiatives Grant – COVID-19 Funding, SAP #4100089371
Publication information: “Design of novel and highly selective SARS-CoV-2 main protease inhibitors,” from Antimicrobial Agents and Chemotherapy.
For a printer-friendly version of this release, please click here.
ABOUT THE WISTAR INSTITUTE:
The Wistar Institute is the nation’s first independent nonprofit institution devoted exclusively to foundational biomedical research and training. Since 1972, the Institute has held National Cancer Institute (NCI)-designated Cancer Center status. Through a culture and commitment to biomedical collaboration and innovation, Wistar science leads to breakthrough early-stage discoveries and life science sector start-ups. Wistar scientists are dedicated to solving some of the world’s most challenging problems in the field of cancer and immunology, advancing human health through early-stage discovery and training the next generation of biomedical researchers. wistar.org