Wistar Scientists Study What Cancer Cells Crave
Wistar’s Zachary Schug and lab are studying the relationship between alcohol intake and some site-specific cancers
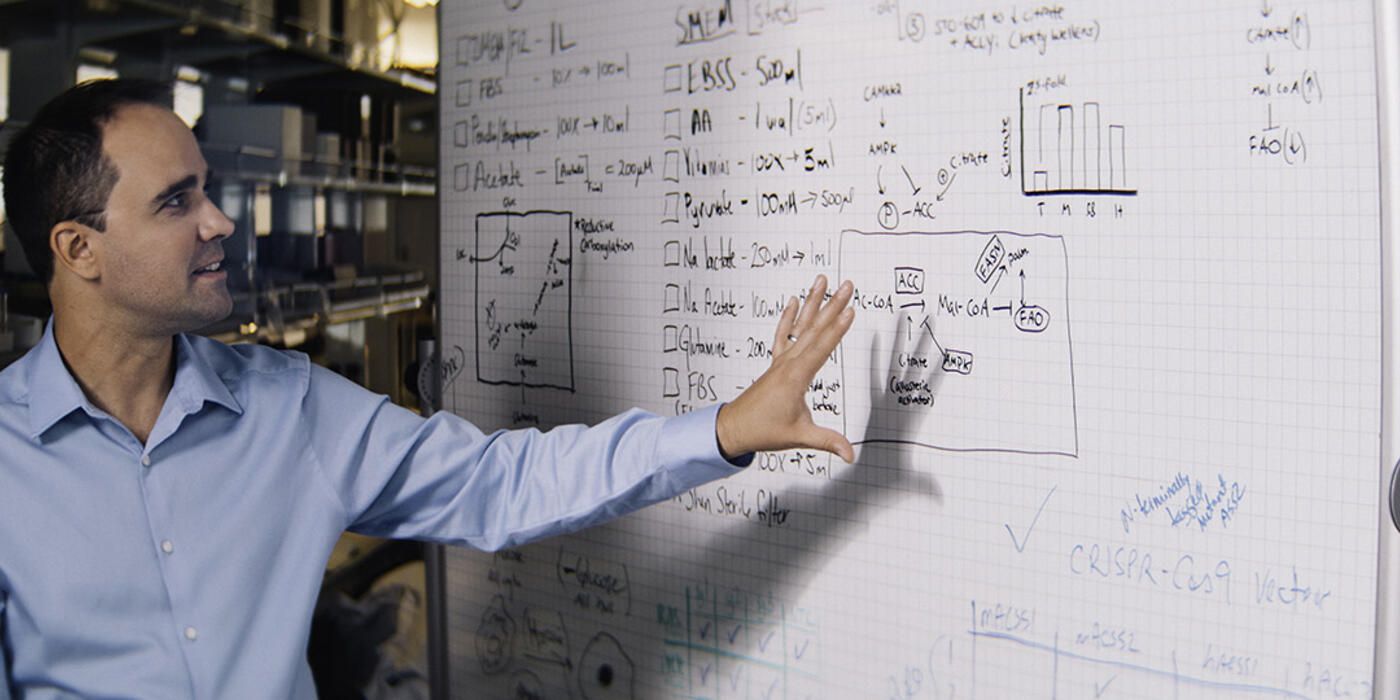
What do cancer cells need to thrive and grow? The Wistar Institute’s Zachary Schug, Ph.D. — assistant professor in the Molecular and Cellular Oncogenesis Program at Wistar’s Ellen and Ronald Caplan Cancer Center — studies how cancer cells’ metabolism works and what cancer cells use for fuel. His lab is on a quest to understand how cancer cells process a nutrient called acetate. We sat down with Dr. Schug to talk about his work in the field of cancer metabolism and its relationship with our daily eating and drinking habits.
What is your lab discovering about what cancer cells need to thrive on and grow?
As researchers who study cancer metabolism, we’re interested in understanding and then hopefully stopping cancer cells from taking advantage of the nutrients available to them to grow and spread. We focus on acetate, a compound that forms when your body processes alcohol; when you drink, acetate is the main thing that alcohol gets broken down into. The liver has a high amount of acetate-metabolizing genes because that’s where our bodies detoxify alcohol.
When you binge drink alcohol, the acetate levels in your blood skyrocket, and acetate goes from being a nobody to a somebody. Both healthy cells and cancer cells can metabolize acetate, depending on the cell type, but we’ve seen certain cancers — including certain breast cancers, melanomas, and even blood cancers — express these acetate-metabolizing genes.
We think drinking alcohol might add fuel to the fire for precancerous or cancerous cells by giving them access to an abundance of acetate. It may accelerate their ability to take advantage of this alternative nutrient source to grow; that’s where we think part of the risk between alcohol and cancer comes from and where we need more research. Cancer adapts and changes over time to survive, so if a cancer is benefitting from acetate metabolism, it can increase the expression of the genes that let it take advantage of that.
My lab works on selectively stopping acetate metabolism in cancer, which we’ve been able to carry out both with gene editing and by designing a small molecule inhibitor with our collaborators as a possible drug candidate.
How does alcohol increase your risk for cancer?
That’s the big question I’m focused on. We know that alcohol correlates with cancer risk. But getting the data to confirm how alcohol puts you at risk, at the molecular level — what we rely on to come up with potential therapies — has been surprisingly difficult.
We have good granular data on alcohol use from people suffering from alcoholism specifically, but the general clinical data on alcohol use for cancer patients — that’s a lot less well characterized. Knowing the difference between someone who has a glass of wine with dinner five nights a week and someone who has five cocktails after work on Friday is especially important for gauging acetate levels, possible health impacts, etc.
We also have concerns about alcohol and acetate in the context of cancer remission. What if there are some cancer cells left behind that are predisposed to using acetate for their growth? Then continuing to drink or even increasing one’s amount of alcohol consumption could be a major risk — one we need to understand better so people can make informed decisions.
We are collaborating with the Philadelphia VA Medical Center and ChristianaCare for cancer patient blood samples, and I’m hopeful that, by collecting detailed survey data on their drinking habits in combination with analyzing their metabolic profiles we’ll be able to get a better understanding of the risk mechanics of alcohol.
Are there other possible health effects from acetate?
We think cancer isn’t the only thing involved in alcohol–acetate signaling. Because many bacteria also produce acetate as a by-product of bacterial metabolism, the immune system, when it sees a spike in acetate levels, can take that as a signal to combat infection. But if someone has chronic exposure to elevated acetate, it becomes a “boy who cried wolf” situation: the immune system starts to get used to higher levels of acetate as a new normal, which we think can put someone at greater risk for infections.
It’s worth considering our dietary and drinking habits carefully in light of realistic risks. This is why granular biomedical research is so important: once we understand what happens at the molecular level — X causes Y, which leads to Z — we have a much better picture of how to assess and combat risks to human health.