Unraveling The Enigmas of Melanoma
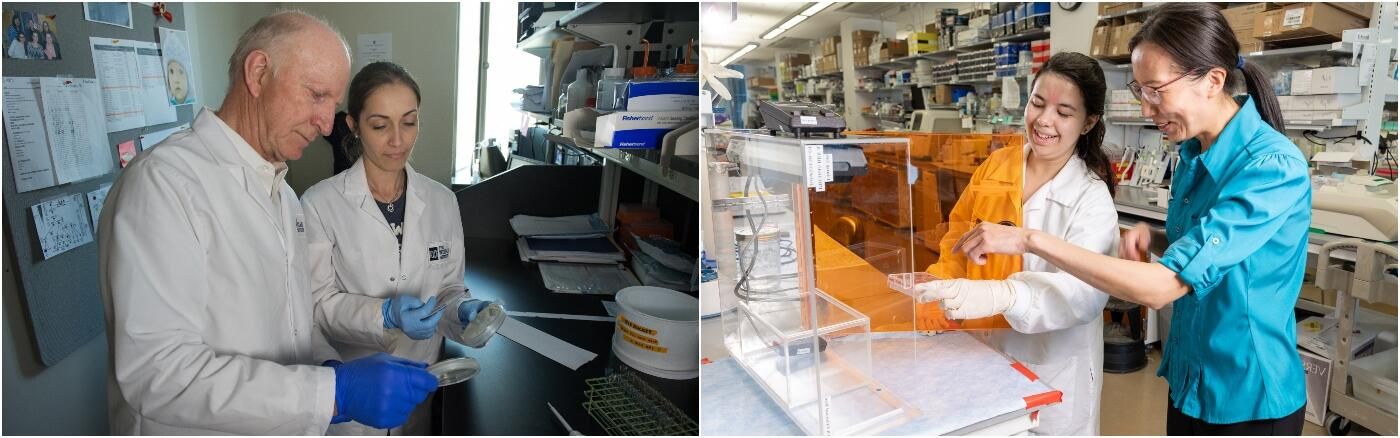
Meenhard Herlyn, D.V.M., D.Sc., is known internationally as one of the fathers of melanoma research. As the founder of The Wistar Institute Melanoma Research Center, he has led the way with breakthrough discoveries about this mysterious and hard-to-treat cancer. A highlight of his work includes building Wistar’s collection of patient-derived xenografts — a groundbreaking tool that allows tumor cells to be implanted into models for melanoma research.
Now, a new generation of melanoma researchers are building on that foundation. This up-and coming-scientific force includes Chengyu Liang, M.D., Ph.D., a rising star in studying how UV exposure damages cells.
“Dr. Herlyn is a great mentor and a great scientist,” Liang said. “He established the platform, the foundation, that has been indispensable not only for Wistar melanoma research, but for the entire melanoma research field.”
BUILDING A BETTER MODEL
“One of the guiding forces in our research has been to mimic human disease, to figure out what makes cells become cancer, and to use this knowledge for new strategies to develop therapies,” Herlyn said.
One of these strategies involves the use of artificial skin. Lab-grown skin had previously been developed for wound healing. Using this existing technology, Herlyn pioneered its application to melanoma research. Herlyn’s team was the first to use artificial skin to grow and study melanocytes — normal pigment cells — which they have used to understand how cancer cells form and how to make treatment more effective.
“We wanted to really know what tumor cells do, and to understand that, we first need to know what normal cells do and where the tumor cells come from,” he explained.
Herlyn joined Wistar in 1976 and spent the early years of his career focused on developing monoclonal antibody treatments, a breakthrough drug that mimics or enhances the immune system’s natural disease fighting activity to attack cancer cells.
One of Herlyn’s frequent collaborators during this time was his wife, Wistar scientist Dr. Dorothee Herlyn, who is now retired. “She was the immunologist of the family,” he said. Together, they helped develop a number of monoclonal antibody molecules, some of which are still used in cancer therapies today.
Herlyn is also behind Wistar’s patient-derived xenograft program which supports a collection of patient cancer tissues. These samples can be implanted into genetically altered mice to more closely mimic conditions in the human body. It’s a powerful tool scientists can use to conduct cancer experiments and test new treatments under conditions that more closely mimic the disease in humans.
“We now have more than 500 tumors from patients,” Herlyn said. “These come directly from the patient and are implanted without ever being cultured, making them much more like real life tumors.”
THE “SUNSCREEN GENE” AND MELANOMA
Dr. Liang didn’t set out to study melanoma. Originally trained as a medical doctor, she became a research scientist with the mission of improving patient outcomes. Her drive to understand cancer and develop better treatments became more personal after her mother passed away following a two-year battle with cancer.
“When someone you love has cancer, you’re trying to find answers. Why did this person have cancer? Why is this treatment not helping?” she said. “Eventually, that drove me to get my Ph.D. in medical science. I wanted to know more.”
Liang initially focused her research on tumor virology, studying how viruses cause cancer. During her research, she encountered a gene called UVRAG that piqued her interest in melanoma.
Previous work had found that this gene seemed to be involved in protecting skin cells from UV radiation, but the mechanism behind it was unclear. Liang’s team showed how the gene repaired DNA damage from UV radiation, and that disrupting the gene could increase a person’s risk of melanoma and other skin cancers. They nicknamed UVRAG the “sunscreen gene.”
The finding sparked many questions about how UV radiation causes genetic mutations that lead to cancer. “The question we asked is, ‘What makes melanoma melanoma?’” she said.
One thing that sets melanoma apart is its extremely high rate of genetic mutations — much higher than other cancers. “It’s in the skin, which is where the body interconnects with the environment and UV radiation, so in a way, that’s not surprising,” she explained.
Liang’s recent research has focused on identifying signs of DNA-repair deficiency as an early sign of damage that can trigger melanoma-driving mutations. “If we can find genetic signs that can predict this process, we might be able to catch the disease much earlier,” she noted.
CULTURING COLLABORATION
Herlyn not only laid the groundwork for Liang and fellow cancer researchers. He also serves as a leader and mentor who is generous with his knowledge and support, Liang said. “He’s like a big dictionary of melanoma,” she described. “When you have a question, he can always share something instructive.”
This philosophy of collaboration, Herlyn shared, has been a driving force in his work. “One of the major strengths at Wistar has been our flexibility and our ability to look for collaborators,” he pointed out. “My approach has always been to look for the best people I could work with.”
This has included a longtime collaboration with oncologists, pathologists, and other clinical colleagues at the University of Pennsylvania as well as other institutions. “I’ve always believed strongly in a good connection between the laboratory and the clinician,” he said.
Herlyn also helped found the Society for Melanoma Research, the first ever medical conference dedicated to bringing together researchers, clinicians, and patients to share knowledge about melanoma. Liang emphasized that with such a complex and unique disease, it’s critical for scientists to work together to find new diagnostic tools and treatments.
“There’s still a lot of mystery,” she stated. “Despite all the tremendous progress we have made in the melanoma field, I think we are still at the tip of the iceberg.”